Selina Concise Chemistry Class 7 ICSE Solutions – Language of Chemistry
Selina Concise ICSE Solutions for Class 7 Chemistry Chapter 5 Language of Chemistry
Points to Remember :
1. A chemical reaction involves the transformation of original substance into an altogether new substance(s).
2. A chemical reaction can be represented with the help of the symbols or the formulae of the elements and the compounds taking part in that reaction. This gives a chemical equation.
3. Certain necessary conditions for a chemical reaction to happen are close contact, solution form, heat, light and catalyst.
4. Characteristics of chemical reactions are change of colour, evolution of a gas, formation of a precipitate, change of state, change of smell and evolution/absorption of heat.
5. A complete chemical equation symbolically represents the reactants, products and their physical states.
6. The substances that react with each other are called reactants and they are represented on the left hand side of the equation. The substances that are formed as a result of the reaction are called products. They are represented on the right hand side of the equation.
7. A chemical equation needs to be balanced to make it follow the law of the conservation of mass.
8. The law of conservation of mass states that mass can be neither created nor destroyed, it can only be transformed from one form to another.
9. A chemical equation gives both qualitative and quantitative information about the reactants and products.
EXERCISE
Question 1.
(a) Define chemical reaction.
(b) What is a chemical equation?
(c) Why do we need to balance chemical equations?
Answer:
(a) Chemical reaction : Any chemical change in matter which involves its transformation into one or more new substances is called a chemical reaction.
(b) Chemical equation : A chemical equation is the symbolic representation of a chemical reaction using the symbols and the formula of the substances involved in the reaction.
(c) A chemical equation needs to be balanced so as to make the number of the atoms of the reactants equal to the number of the atoms of the products.
Question 2.
State four conditions necessary for chemical reactions to take place.
Answer:
Conditions necessary for chemical reactions :
1. Close contact
2. Solution form
3. Heat
4. Light
5. Catalyst
3. Differentiate between :
(a) Reactants and products.
Reactants Products
1. The substances that react with one 1. The new substances formed are called
another are called reactants. products.
2. Reactants are written on the left 2. Products are written on the right hand hand side of equation side of equation.
(b) Chemical reaction and chemical equation.
Chemical reaction Chemical Equation
Any chemical change in matter which involves A chemical equation is the symbolic its transformation into one or more new representation of a chemical reaction using the
substances is called a chemical reaction . symbols and the formula of the substances involved in the reaction.
(c) A balanced and a skeletal chemical equation.
Balanced Equation Skeletal Equation
A balanced chemical equation is one in which In a skeletal equation the number of atoms
the number of atoms each element on the reactant on reactant side are not equal to number of
side is equal to the number of atoms of atoms of product side.
that element on the product side.
Question 4.
Write word equations for the following skeletal equations:
(a) KClO3 → KCl + O2
(b) Zn + HCl → ZnCl2 + H2
(c)FeCl2 + Cl2 → FeCl3
(d) CO + O2 → CO2
(e) Ca + O2 → CaO
(f) Na + O2 → Na2O
(g) NaOH + H2SO4 → Na2SO4 + H2O
(h) AgBr → Ag + Br2
(i) KNO2 → KNO2 + O2
Answer:
(a) 2KClO3 → 2KCl+ 3O2
(b) Zn + 2HCl → ZnCl2 + H2
(c) 2FeCl2 + Cl2 → 2FeCl3
(d) 2CO + O2 → 2CO2
(e) 2Ca + O2 → 2CaO
(f) 4Na + O2 → 2Na2O
(g) 2NaOH + H2SO4 → Na2SO4 + 2H2O
(h) 2AgBr → 2Ag + Br2
(i) 2KNO3 → 2KN02 + O2
Question 5.
Balance the following chemical equations :
(a) FeS + HCl → FeCl2 + H2S
(b) Na2CO3 + HCl → NaCI + H2O + CO2
(c) H2 + O2 → H2O
(d) Na20 + H20 → NaOH
Answer:
(a) FeS + 2HCl → FeCl2 + H2S
(b) Na2CO3 + 2HCl → 2NaCl + H2O + CO2
(c) 2H2+ O2 → 2H2O
(d) Na2O + H2O → 2NaOH
Question 6.
What information do you get from the equation H2+ Cl2 → 2HCl ?
Answer:
(a)Hydrogen and chlorine molecules are the reactants.
(b)They are in gaseous form.
(c)The product is hydrogen chloride gas.
(d)Two molecules of hydrogen chloride are formed.
Question 7.
Write your observations for the following chemical reactions and name the products formed :
(a) When sugar is heated.
(b) When manganese dioxide is added to potassium chlorate and heated.
(c) When dilute acetic acid is poured on baking soda.
(d) When an aqueous solution of sodium chloride is mixed with an aqueous solution of silver nitrate.
(e) When ammonium chloride is heated with sodium hydroxide.
(f) When water is added to quick lime?
Answer:
(a) Black solid mass (charcoal) is formed along with water vapours.
(b) Manganese dioxide acts as a catalyst for the decomposition of potassium chlorate into potassium chloride and oxygen at a lower temperature.
(c) Sodium acetate, CO2 and water is formed.
(d) A white insoluble solid precipitate of silver chloride is formed along with Sodium nitrate.
(e) When solid ammonium chloride is heated with sodium hydroxide solution, a gas ammonia is evolved which is recognised by its strong pungent smell.
(f) When water is added to quick lime, a large amount of heat energy is evolved.
Question 8.
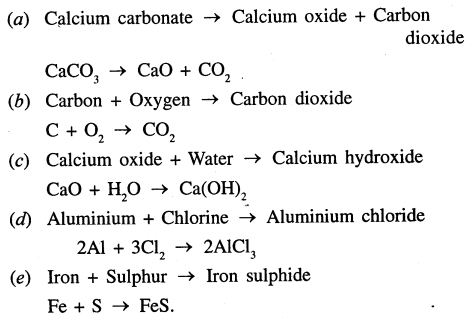
Write symbolic representation for the following word equations and balance them :
(a) Calcium carbonate → Calcium oxide + Carbon dioxide
(b) Carbon + Oxygen → Carbon dioxide
(c) Calcium oxide + Water → Calcium hydroxide
(d) Aluminium + Chlorine → Aluminium chloride
(e) Iron + Sulphur → Iron sulphide
Answer:
OBJECTIVE TYPE QUESTIONS
1. Fill in the blanks:
(a) The substances which undergo chemical change are called reactants.
(b) The substances formed as a result of a chemical reaction are called products.
(c) During a chemical reaction transfer of energy takes place.
(d) The basic conditions necessary for a chemical reaction is close contact.
(e) In some chemical reactions an insoluble precipitate is formed when two solutions are mixed.
2. Write ‘true’ or ‘false’ for the following statements :
(a) No new substance is formed during a chemical reaction : True
(b) Hydrogen sulphide has rotten egg smell : True
(c) When potassium iodide solution is added to lead acetate solution a red precipitate is formed : False
(d) A black residue is formed when sugar is heated : True
(e) When iron and sulphur are heated together a grey mass is formed which is attracted by a magnet : False
(f) A chemical equation gives only qualitative information of a chemical reaction : False
MULTIPLE CHOICE QUESTIONS
1. A chemical equation is a statement that describes a chemical change in terms of
(a) symbols and formulae
(b) energy
(c) number of atoms
(d) colours
2. Balancing a chemical equation is based on
(a) Law of conservation of mass
(b) Mass of reactants and products
(c) Symbols and formulae
(d) None of the above
3. Copper carbonate when heated, it turns :
(a) Blue
(b) Green
(c) Black
(d) Yellow