Selina ICSE Solutions for Class 9 Chemistry Chapter 3 Water
Exercise 3(A)
1. Water exists in all three states. Discuss.
Solution 1.
In free state, water occurs in three states viz, solid, liquid and gaseous state.
- Solid state of water: In solid state, a large amount of fresh water is found in the form of snow or Ice.
- Liquid state of water: Most of the water present in oceans, on land water found in streams, rivers, lakes, ponds, springs are also liquid state of water.
- Gaseous state of water: In gaseous form, water vapours present in air. When these vapours condense, they form clouds, mist, fog etc. are examples of water in gaseous form.
2. Why is water considered a compound?
Solution 2.
Water is considered as a compound because it is made up of two elements, hydrogen and oxygen combined in the ratio of 1 : 8 by mass.
Mass ratio of elements H2O
H : O, 2 X 1 : 16 X 1 = 1 : 8
(Atomic mass of H = 1, O = 16)
And also components of water cannot be separated by physical methods but can be separated by electrolysis of water.
3. Why does temperature in Mumbai and Chennai not fall as low as it does in Delhi?
Give the properties of water responsible for controlling the temperature of our body.
3. Why does temperature in Mumbai and Chennai not fall as low as it does in Delhi?
Give the properties of water responsible for controlling the temperature of our body.
Solution 3.
(a) The temperature in Mumbai and Chennai do not fall as low as in Delhi because Mumbai and Chennai are situated at the bank of the oceans due to high specific heat capacity the presence of a large amount of water is able to modify the climate of the nearby land areas are warmer in winter and cooler in summer temperature does not fall wherever Delhi has not same condition.
(b) Our body is almost 65% of water and water has property of specific heat. Due high specific heat capacity the presence of large amount of water is able to modify the climate of the body and control the temperature of our body which is warm in winter and cooler in summer.
4.'Water is the universal solvent'. Comment.
4.'Water is the universal solvent'. Comment.
Solution 4.
Water dissolves many substances forming aqueous solution. It can dissolve solids, liquids and gases. When a solid dissolves in water the solid is said to be solute, the water (the solvent) and the resultant liquid, the solution. So it is said that water is a universal solvent. In other words water can dissolve nearly every substance.
5.What causes the violence associated with torrential rain?
5.What causes the violence associated with torrential rain?
Solution 5.
The sudden release of the latent heat of condensation causes the violence associated with torrential rain.
6.(a) Which property of water enables it to modify the climate ?
(b)How do fishes and aquatic animals survive when the pond gets covered with thick ice?
(c) What is the effect of impurities present in water on melting point and boiling point of water?
6.(a) Which property of water enables it to modify the climate ?
(b)How do fishes and aquatic animals survive when the pond gets covered with thick ice?
(c) What is the effect of impurities present in water on melting point and boiling point of water?
Solution 6.
(a) Specific heat
(b) Water has an unusual physical property. When cooled, it first contracts in volume, as do other liquids, but at 4°C (maximum density), it starts expanding, and continues to do so till the temperature reaches 0°C, the point at which it freezes into ice.
The property of anomalous expansion of water enables marine life to exist in the colder regions of the world, because even when the water freezes on the top, it is still liquid below the ice layer.
(C) Melting point: The constant temperature at which a solid changes into a liquid state, by absorbing the heat energy is called melting point.
Boiling point: The temperature at which water starts boiling under normal pressure is called boiling point of water.” It is 100°C. Boiling point increases with increase in pressure and vice versa.”
Specific heat capacity: The amount of heat required to raise the temperature of unit mass of that substance through 1°C.
Latent heat of vaporization of water: The energy required to change water into its vapour at its boiling point without any change in temperature is called latent heat of vaporization of water.
Latent heat of vaporization of water is 2260 joules J/g or 540 cal/g.
In the reverse process, 2260 joules of heat is released when 1 g of steam condenses to form 1 g of water at 100°C.
Latent heat of fusion of ice: The amount of heat energy required by ice to change into water is called latent heat of fusion of ice.
Latent heat of fusion of ice is 336 J/g or 80 cal/g.
In the reverse process, 336 joules of heat is released when 1 g of water solidifies to form 1 g of ice at 0°C.
7.What is the composition of water? In what volume its elements combine?
7.What is the composition of water? In what volume its elements combine?
Solution 7.
Composition of water :- Hydrogen and Oxygen Volume Ratio = H2 : O
= 2 : 1
8.The properties of water are different from the properties of the elements of which it is formed. Discuss.
8.The properties of water are different from the properties of the elements of which it is formed. Discuss.
Solution 8.
The properties of water are different from the properties of elements from which it is formed
9.How is aquatic life benefited by the fact that water has maximum density at 4oC?
Solution 9.
The property of anomalous expansion of water enables aquatic life to exist because of the water freezes on top of the surface of the water body, but it is still liquid below the ice layer.
10.
10.
Solution 10.
(a) Aim: To show that tap water contains dissolved salts.
Procedure: Put some tap water on a clean watch glass and place it over a beaker containing water as shown in fig. Boil the water in the beaker. When all the water has evaporated from the watch glass, remove the burner and let it cool. We see at the watch glass against light, a number of concentric rings of solids matter on it. These are dissolved impurities, left behind after evaporation of water. To show that water contains dissolved solids.
(b) Aim: To show that tap water contains dissolved gases.
Procedure: Take a round bottomed flask and filled it with the tap water. In its mouth fix a delivery tube, in such a way that its lower end of the delivery tube is in line with the under – surface of the cork.
Arrange the apparatus according to diagram.
Heat the flask with the help of a Bunsen burner. It is seen that tiny bubbles of gas are coming out, which stick to the sides of flask, heat it continuously, till the water is about to boil. It is seen that Bubbles of gas start coming out of beehive shelf.
Now lower the flame, to keep the water just near its boiling point. Invert over the beehive shelf a graduated tube, completely filled with tap water. Gradually, the boiled off air, starts collecting in the flask. Collect at least two tubes of boiled off air.
11.State the importance of the suitability of CO2 and O2 in water.
Solution 11.
CO2 and O2 add taste to water for drinking purposes.
12.How is air dissolved in water different from ordinary air?
12.How is air dissolved in water different from ordinary air?
Solution 12.
Oxygen is more soluble in water than nitrogen. Air dissolved in water contains a higher percentage of oxygen. That is, 30% – 35% and in ordinary air it is only 21 %. In this way air dissolved in water is different from ordinary air.
13.Rivers and lakes do not freeze easily?
13.Rivers and lakes do not freeze easily?
Solution 13.
Rivers and lakes have large amount of water and water has high specific heat capacity, due to which they do not freeze easily.
Even if they freeze, they freeze at top layer. There is water below due to Anomalous expansion of water.
14.What is the importance of dissolved salts in water?
14.What is the importance of dissolved salts in water?
Solution 14.
Importance of dissolved salts in water:
- Dissolved salts provide a specific taste to water.
- Dissolved salts act as micro-nutrients for the growth and development of living beings.
15.Explain why:
Boiled or distilled water tastes flat.
Ice at zero degrees centigrade has greater cooling effect than water at 0oC.
Burns caused by steam are more severe than burns caused by boiling water.
Rain water does not leave behind concentric rings when boiled.
Air dissolved in water contains a higher proportion of oxygen.
If distilled water is kept in a sealed bottle for a long time, it leaves etchings on the surface of the glass.
Boiled or distilled water tastes flat.
Ice at zero degrees centigrade has greater cooling effect than water at 0oC.
Burns caused by steam are more severe than burns caused by boiling water.
Rain water does not leave behind concentric rings when boiled.
Air dissolved in water contains a higher proportion of oxygen.
If distilled water is kept in a sealed bottle for a long time, it leaves etchings on the surface of the glass.
Solution 15.
(a) Boiled water tastes flat because boiled water does not contain matter like air, carbon dioxide and other minerals, So the boiled water tastes flat.
(b) Ice at zero degree centigrade gives more cooling effect than water at 0°C because, ice at 0°C absorbs 336J per gram of energy to melt to 0°C water and hence gives more cooling effect.
(c) Burn caused by steam is more severe than burn caused by boiling water because, 1 g of steam contains 2268J more energy than 1 g of boiling water and hence, cause more severe burns.
(d) Rain water does not leave concentric rings when boiled because rain water does not contain dissolved solid, so it does not form concentric rings.
(e) Air dissolved in water contains a higher percentage of oxygen because, solubility of oxygen in water is more than in air. So, air dissolved in water contains a higher percentage of oxygen.
(f) If distilled water is kept in a sealed bottle for a long time, it leaves etching on the surface of glass because, the substances which are insoluble in water, actually dissolve in minute traces in water. Even when we drink water from a glass, an extremely small amount of glass dissolves in water, so we see the etching on the surface of glass when a long time sealed bottle of distilled water poured into the glass.
16.Explain what you understand from the following diagram:
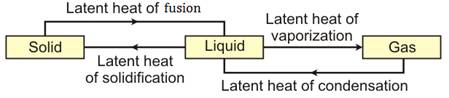
16.Explain what you understand from the following diagram:

Solution 16.
(i) When solid changes with liquid, it absorbs heat equal to latent heat of fusion and when a liquid changes into solid, it loses heat equal to latent heat of solidification.
(ii) When a liquid changes into gas, it absorbs heat equal to latent heat of vaporization and when a gas condenses into liquid, it loses heat equal to latent heat of condensation.
Exercise 3(B)
1.Explain the terms:
Solution
Solute
Solvent
1.Explain the terms:
Solution
Solute
Solvent
Solution 1.
(a) Solution: Solution is a homogeneous mixture of two or more substances, components of which cannot be seen separately.
(b) Solute: A solute is the substance that dissolves in a solvent to form a solution.
(c) Solvent: A solvent is a medium in which the solute dissolves.
Solution = Solute + Solvent
2.Explain why a hot saturated solution of potassium nitrate forms crystals as it cools.
2.Explain why a hot saturated solution of potassium nitrate forms crystals as it cools.
Solution 2.
The solubility of nitrate decreases with the fall in temperature. Thus, when saturated solution of nitrate is cooled the excess of it separates from solution, in the form of crystals.
3.Give three factors which affect the solubility of a solid solute in a solvent.
3.Give three factors which affect the solubility of a solid solute in a solvent.
Solution 3.
The three factors on which the solubility of a solid depends are:
1. Temperature
2. Nature of the solid
3. Nature of solvent
4.(a) If you are given some copper sulphate crystals, how would you proceed to prepare its saturated solution at room temperature?
(b) How can you show that your solution is really saturated?
4.(a) If you are given some copper sulphate crystals, how would you proceed to prepare its saturated solution at room temperature?
(b) How can you show that your solution is really saturated?
Solution 4.
Take 100 g of distilled water in a beaker. To this add one gram of copper sulphate crystals.
Stir this mixture with the help of a glass rod and dissolve copper sulphate crystals. Similarly, go on dissolving more of copper sulphate, (1 gram) at a time with constant and vigorous stirring. A stage is reached when no more copper sulphate dissolves. It is called saturated solution at this temperature.
Take this saturated solution of copper sulphate some solution in a test tube and add some copper sulphate crystals. The crystals do not dissolve but settle down. This indicates that the solution is really saturated.
5.Define (i) Henry's law and (ii) Crystallisation.
State the different methods of crystallisation.
5.Define (i) Henry's law and (ii) Crystallisation.
State the different methods of crystallisation.
Solution 5.
(a) (i) Henry’s law: It states that at any given temperatures, the mass of a gas dissolved in a fixed volume of a liquid or solution is directly proportional to the pressure on the surface of a liquid.
(ii) Crystallisation: It is the process by which crystals of a substance separate out on cooling its hot saturated solution.
(b) In the laboratory, crystals may be obtained by the following methods:
1. By cooling a hot saturated solution gently.
2. By cooling a fused mass.
3. By sublimation.
4. By evaporating slowly a saturated solution.
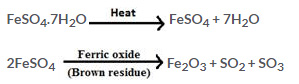
6.What would you observe when crystals of copper (II) sulphate and iron (II) sulphate are separately heated in two test tubes?
6.What would you observe when crystals of copper (II) sulphate and iron (II) sulphate are separately heated in two test tubes?
Solution 6.
Action of heat on copper (II) sulphate crystals
When copper (II) sulphate crystals are heated in a hard glass test tube, the following observations are made.
1. The crystals are converted into powdery substance.
2. The crystals lose their blue coloration on further heating.
3. Steaming vapour are produced inside the tube which condense near the mouth of the tube to form a colourless liquid.
4. On further heating, steams escapes from the mouth of the tube and water gets collected in a beaker placed under the mouth of tube.
5. On further heating, the residue changes to a white powder and steam stops coming out.
Action of heat on iron (II) sulphate
When iron (II) sulphate is heated in a test tube, the following observations are made.
1. The crystals crumble to white powder and a large amount of steam and gas are given out.
2. On strong heating, a brown residue of ferric oxide (Fe2O3) is produced and a mixture of SO2 and SO3 is given off.
7.
Solution 7.
Table salt becomes sticky on exposure during the rainy season because, table salt generally contains a small percentage of Magnesium chloride, as an impurity. Since, these impurities absorb moisture from air due to their deliquescent nature; therefore it gets wet in rainy season and becomes sticky.
8.What is the effect of temperature on solubility of KNO3 and CaSO4 in water?
Solution 8.
Potassium nitrate (KNO3): Increase in solubility of substances with rise in temperature.
Calcium sulphate (CaSO4): Decrease in solubility of substances with rise in temperature.9.Solubility of NaCl at 40oC is 36.5 g. What is meant by this statement?
Solution 9.
Solubility of NaCl at 40°C is 36.5 g means 36.5 g of NaCl dissolves in 100 g of water at the temperature of 40°C.
10.Which test will you carry out to find out if a given solution is saturated or unsaturated or supersaturated?
10.Which test will you carry out to find out if a given solution is saturated or unsaturated or supersaturated?
Solution 10.
1. A solution in which more of solute can be dissolved at a given temperature is an unsaturated
solution.
2. A solution in which no more solute can be dissolved at a given temperature is a saturated solution at that temperature.
3. A solution in which some solute separates on cooling slightly is a super saturated solution.
11.What is the effect of pressure on solubility of gases? Explain with an example.
11.What is the effect of pressure on solubility of gases? Explain with an example.
Solution 11.
• With the increase in pressure the solubility of a gas in water increases.
• With the increase in temperature, the solubility of a gas in water decreases.
• For example, The solubility of carbon dioxide in water under normal atmospheric pressure is low, but when the water surface is subjected to higher pressure, a lot more of CO2 gas gets dissolve in it.
• Similarly, In the case of soda water, on opening the bottle, the dissolved gas rapidly bubbles out, since the pressure on the surface of the water suddenly decreases.
12.
12.
Solution 12.
A solubility curve is a line graph which shows changes in the solubility of a solute in a given solvent with a change in temperature.
To obtain this curve, values of temperature are plotted on X-axis and values of solubility on Y-axis.
Applications:
1. The variation in the solubility of any given substance with temperature can be studied with the help of solubility curve.
2. To compare the solubility of different substances, at a given temperature.13.Explain why:
Water is an excellent liquid to use in cooling systems.
A solution is always clear and transparent.
Lakes and rivers do not suddenly freeze in the winters.
The solute cannot be separated from a solution by filtration.
Fused CaCl2 or conc. H2SO4 is used in a desiccator.
Effervescence is seen on opening a bottle of soda water.
Table salts become sticky on exposure to humid air during the rainy season.
Solution 13.
(a) Water is an excellent liquid to use in cooling systems due to its ability to absorb large quantities of heat i.e. specific heat = 4.2J/goC, so it is used in cooling system i.e. cooling agent.
(b) A solution is always clear and transparent because in a solution, solid disappeared in water and water has property – cleanliness and transparent. So, the solution is always clean and transparent.
(c) Lakes and rivers do not freeze suddenly in winters due to high specific latent heat of solidification. i.e. the amount of heat released when 1 g of water solidifies to form 1 g of ice at 0°C. It is about 336 J/g or 80 cal/g. Such enormous amount of heat leads to immediate freezing of lakes and rivers in winter.
(d) The component that actually dissolves in a solvent is known as solute. So it can separated from solution by filtration process. But filtration process is applicable only when solute is insoluble in solution. So the solute cannot be separated from solution by filtration.
(e) Fused CaCl2 or concentrated H2SO4 is deliquescent in nature absorbs moisture and hence, these are used in desiccators or as drying agent.
(f) Carbon dioxide is dissolved in soda water under pressure. On opening the bottle, the pressure on the surface of water suddenly decreases, therefore, the solubility of CO2 in water decreases and the gas rapidly bubbles out.
14.Normally, solubility of crystalline solid increases with temperature. Does it increase uniformly in all cases? Name a substance whose solubility:
Increases rapidly with temperature.
Increases gradually with temperature.
Increases slightly with temperature.
Initially increases then decreases with rise in temperature.
14.Normally, solubility of crystalline solid increases with temperature. Does it increase uniformly in all cases? Name a substance whose solubility:
Increases rapidly with temperature.
Increases gradually with temperature.
Increases slightly with temperature.
Initially increases then decreases with rise in temperature.
Solution 14.
(a) Potassium nitrate
(b) Potassium chloride
(c) Sodium chloride
(d) Calcium sulphate
15.What are drying or desiccating agents? Give examples.
15.What are drying or desiccating agents? Give examples.
Solution 15.
These are the substances which can readily absorb moisture from other substances without chemically reacting with them.
For example,
Phosphorus pentoxide (P2O5), quick lime (CaO).
16. Complete the following table:
16. Complete the following table:
Common Name
|
Chemical Name
|
Formula
|
Acid, base or salt
|
Efflorescent,
hygroscopic or deliquescent substance
|
Solid caustic potash
| ||||
Quick lime
| ||||
Oil of vitriol
| ||||
Washing soda
| ||||
Solid caustic soda
| ||||
Blue vitriol
|
Solution 16:
17.In which of the following substances will there be
Increase in mass
Decrease in mass
No change in mass when they are exposed to air?
Sodium chloride
Iron
Conc. sulphuric acid
Table salt
Sodium carbonate crystals
Increase in mass
Decrease in mass
No change in mass when they are exposed to air?
Sodium chloride
Iron
Conc. sulphuric acid
Table salt
Sodium carbonate crystals
Solution 17.
(a) Increase in mass- Iron and conc. sulphuric acid
(b) Decrease in mass- Sodium carbonate crystals
(c) No change in mass- Sodium chloride
18.
Solution 18.
Solution 19.
20.
Solution 20.
(b) Solubility of solid decreases with fall in temperature. A saturated solution on cooling, a part of dissolved solute separates out in the form of crystals.
(c) Solubility of salt at 293K
KNO3 → 32g
NaCl → 36g
KCl → 35g
NH4Cl → 37 g
(d) At 283K lowest solubility is of KNO3 → 21g
(e) Solubility of most of solids usually increases and of gas and liquid always decreases with rise in temperature.
21.
21.
Solution 21.
(a) Wt. of empty dish = 50 gm
Wt. of dish and solution = 65 gm
Wt. of dish and residue = 54.3 gm
Wt. of saturated solution = 65 – 50 = 15 gm
Wt. of crystals = 54.3 – 50 = 4.3 gm
Wt. of water in saturated solution = 15 – 4.3 = 10.7 gm
(b) Wt. of water = 50 gm
Solubility at 500oC = 114 gm
Solubility at 30oC = 86 gm
Solubility from 50oC to 30oC = 114 – 86 = 28 gm.
[Since, Wt. of water = Wt. of solution – Wt. of crystals]
50 g = Wt. of solution – Wt. of crystals
50 + 14 = Wt. of solution
Wt. of solution = 64 gm
Wt. of sodium = Wt. of saturated solution – Wt. of water
= 64 – 50 = 14 gm
Wt. of sodium = 14 gm
22.
Solution 22.
Exercise 3(C)
1.What do you understand bySoft water
Hard water
Temporary hard water
Permanent hard water
Solution 1:
Water is said to be soft when the water containing sodium salts easily gives lather with soap.
Water is said to be hard when it does not readily form lather with soap.
Water which contains only hydrogen carbonates of calcium and magnesium is called temporary hard water.
Water containing sulphates and chlorides of magnesium and calcium is called permanent hard water.
2.What are the causes for
- Temporary hardness
- Permanent hardness
Solution 2:
The presence of hydrogen carbonates of calcium and magnesium makes water temporarily hard.
The presence of sulphates and chlorides of magnesium and calcium makes water permanently hard.3. What are the advantages of (i) soft water and (ii) hard water?
Solution 3:
Advantages of soft water:
When the water is soft, you use much less soap and fewer cleaning products. Your budget will reflect your savings.
Plumbing will last longer. Soft water is low in mineral content and therefore does not leave deposits in the pipes.
Clothes last longer and remain bright longer if they are washed in soft water.
Advantages of hard water:
Water free from dissolved salts has a very flat taste. The presence of salts in hard water makes it tasty. So, hard water is used in making beverages and wines.
Calcium and magnesium salts present in small amounts in hard water are essential for bone and teeth development.
Hard water checks the poisoning of water by lead pipes. When these pipes are used for carrying water, some lead salts dissolve in water to make it poisonous. Calcium sulphate present in hard water forms insoluble lead sulphate in the form of a layer inside the lead pipe and this checks lead poisoning.
4.What are stalgmites and stalactites? How are they formed?
4.What are stalgmites and stalactites? How are they formed?
Solution 4:
In some limestone caves, conical pillar-like objects hang from the roof and some rise from the floor. These are formed by water containing dissolved calcium hydrogen carbonate continuously dropping from the cracks in the rocks. Release of pressure results in the conversion of some hydrogen carbonate to calcium carbonate.
Ca(HCO3)2 → CaCO3 + CO2 + H2O
This calcium carbonate little by little and slowly deposit on both roof and floor of the cave.
The conical pillar which grows downwards from the roof is called stalactite and the one which grows upward from the floor of the cave is called stalagmite.
These meet after a time. In a year, some grow less than even a centimetre, but some may be as tall as 100 cm.
CaCO3 + CO2 + H2O → Ca(HCO3)2
MgCO3 + CO2 + H2O → Mg(HCO3)2
If the water flows over beds of gypsum (CaSO4.2H2O), a little bit of gypsum gets dissolved in water and makes it hard.
5. Name the substance which makes water (i) temporarily hard and (ii) permanently hard.
5. Name the substance which makes water (i) temporarily hard and (ii) permanently hard.
Solution 5:
Hydrogen carbonates of calcium and magnesium
Sulphates and chlorides of magnesium and calcium
6.Give equations to show what happens when temporary hard water is
Boiled
Treated with slaked lime
6.Give equations to show what happens when temporary hard water is
Boiled
Treated with slaked lime
Solution 6:
7.State the disadvantages of using hard water.
Solution 7:
It is more difficult to form lather with soap.
Scum may form in a reaction with soap, wasting the soap.
Carbonates of calcium and magnesium form inside kettles. This wastes energy whenever you boil a kettle.
Hot water pipes ‘fur up’. Carbonates of calcium and magnesium start to coat the inside of pipes which can eventually get blocked.
8.What is soap? For what is it used?
8.What is soap? For what is it used?
Solution 8:
Soap is chemically a sodium salt of stearic acid (an organic acid with the formula C17H35COOH) and has the formula C17H35COONa.
Soap is used for washing purposes.
9.What is the advantage of a detergent over soap?
9.What is the advantage of a detergent over soap?
Solution 9:
Detergents are more soluble in water than soap and are unaffected by the hardness of water as their calcium salts are soluble in water.
10.Why does the hardness of water render it unfit for use in a (i) boiler and (ii) for washing purposes.
10.Why does the hardness of water render it unfit for use in a (i) boiler and (ii) for washing purposes.
Solution 10:
Steam is usually made in boilers which are made of a number of narrow copper tubes surrounded by fire. As the cold water enters these tubes, it is immediately changed into steam, while the dissolved solids incapable of changing into vapour deposit on the inner walls of the tubes. This goes on and makes the bore of the tubes narrower. The result is that less water flows through the tubes at one time and less steam is produced. When the bore of the tube becomes very narrow, the pressure of the steam increases so much that at times the boiler bursts.
If hard water is used, calcium and magnesium ions of the water combine with the negative ions of the soap to form a slimy precipitate of insoluble calcium and magnesium usually called soap curd (scum).
Formation of soap curd will go on as long as calcium and magnesium ions are present. Till then, no soap lather will be formed and cleaning of clothes or body will not be possible. Moreover, these precipitates are difficult to wash from fabrics and sometimes form rusty spots if iron salts are present in water.
11.Explain with equation, what is noticed when permanent hard water is treated with
Slaked time
Washing soda
11.Explain with equation, what is noticed when permanent hard water is treated with
Slaked time
Washing soda
Solution 11:
12.Explain the permutit method for softening hard water.
Solution 12:
Permutit is an artificial zeolite. Chemically, it is hydrated sodium aluminium orthosilicate with the formula Na2Al2Si2O8.XH2O. For the sake of convenience, let us give it the formula Na2P.
A tall cylinder is loosely filled with lumps of permutit. When hard water containing calcium and magnesium ions percolates through these lumps, ions exchange. Sodium permutit is slowly changed into calcium and magnesium permutit, and the water becomes soft with the removal of calcium and magnesium ions.
When no longer active, permutit is regenerated by running a concentrated solution of brine over it and removing calcium chloride formed by repeated washing.
CaP + 2NaCl → Na2P + CaI2
Selina ICSE Solutions for Class 9 Chemistry Chapter 3 Water