Selina ICSE Solutions for Class 9 Chemistry Chapter 1 - The Language of Chemistry
Exercise 1(A)
1. What is a symbol? What information does it convey?
Solution 1.
A symbol is the short form which stands for the atom of a specific element or the abbreviations used for the names of elements.
- It represents a specific element.
- It represents one atom of an element.
- A symbol represents how many atoms are present in its one gram (gm) atom.
- It represents the number of times an atom is heavier than one atomic mass unit (amu) taken as a standard.
2. Why is the symbol S for Sulphur, but Na for Sodium and Si for Silicon.
Solution 2.
In most cases, the first letter of the name of the element is taken as the symbol for that element and written in capitals (e.g. for sulphur, we use the symbol S). In cases where the first letter has already been adopted, we use a symbol derived from the Latin name (e.g. for sodium/Natrium, we use the symbol Na). In some cases, we use the initial letter in capital together with a small letter from its name (e.g. for silicon, we use the symbol Si).
3.Write the full form of IUPAC. Name the elements represented by the following Symbols: Au, Pb,Sn,Hg
Solution 3.
The full form of IUPAC is International Union of Pure and Applied Chemistry.
Names of the elements:
Au – Gold
Pb – Lead
Sn – Tin
Hg – Mercury
4. If the symbol for cobalt, Co were written as CO, What would be wrong with it.
4. If the symbol for cobalt, Co were written as CO, What would be wrong with it.
Solution 4.
Co stands for Cobalt. If we write CO, then it would mean that it is a compound containing two non-metal ions, i.e. carbon and oxygen, which forms carbon monoxide gas.
5. What do the following symbols stand for?
a) H b) H2 c) 2H
a) H b) H2 c) 2H
Solution 5.
(a) H stands for one atom of hydrogen.
(b) H2 stands for one molecule of hydrogen.
(c) 2H stands for two atoms of hydrogen.
6. What is meant by atomicity? Name a diatomic element.
6. What is meant by atomicity? Name a diatomic element.
Solution 6.
The number of atoms of an element that join together to form a molecule of that element is known as its atomicity. Diatomic molecules: H2, O2, N2, Cl2
7.(a) Explain the terms valency and variable valency
Solution 7 (a)
- Valency of Na is +1 because it can lose one electron.
- Valency of O is -2 because it can accept two electrons.
Variable valency: It is the combining capacity of an element in which the metal loses more electrons from a shell next to a valence shell in addition to electrons of the valence shell.
7.(b) How are the elements with variable valency named? Explain with an example.
7.(b) How are the elements with variable valency named? Explain with an example.
Solution 7 (b)
If an element exhibits two different positive valencies, then
- for the lower valency, use the suffix -OUS at the end of the name of the metal
- for the higher valency, use the suffix -IC at the end of the name of the metal.
Example:
8. Give the formula and valency of
a) aluminate
b) chromate
c) aluminium
d) cupric
Solution 8.
a) aluminate
b) chromate
c) aluminium
d) cupric
Solution 8.
Solution 9.
Chemical formula: The chemical formula of a substance (element or compound) is a symbolic representation of the actual number of atoms present in one molecule of that substance.
It also indicates the fixed proportion by weight in which atoms combine.
Rules:
(i) The positive and negative radicals are written side by side (+ve first) with their charge as a superscript on the right side.
(ii) Charges are then interchanged and written as a subscript.
(iii) The final formula is written without the sign of charge, e.g. Hg2O
10. What do you understand by following terms?
a) Acid radical
Solution 10 (a)
a) Acid radical
Solution 10 (a)
Acid radical: The electronegative or negatively charged radical is called an acid radical.
Examples: Cl–, O2-
b) Basic radical
Solution 10 (b)
Basic radical: The electropositive or positively charged radical is called a basic radical.
Examples: K+, Na+
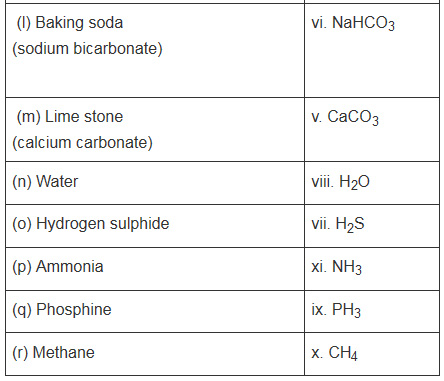
11. Match the following
| Compound | Formula |
| (a) Boric acid | NaoH |
| (b) Phosphoric acid | SiO2 |
| (c) Nitrous acid | Na2CO3 |
| (d) Nitric acid | KOH |
| (e) Sulphorous acid | CaCO3 |
| (f) Sulphuric acid | NaHCO3 |
| (g) Hydrochloric acid | H2S |
| (h) Silica (Sand) | H2O |
| (i) Caustic soda ( Sodium Hydroxide) | PH3 |
| (j) Caustic potash( potassium hydroxide) | CH4 |
| (k) Washing soda( Sodium carbonate) | NH3 |
| (l) Baking Soda ( Sodium bi carbonate) | HCl |
| (m) Lime stone ( calcium carbonate) | H2SO3 |
| (n) Water | HNO3 |
| (o) Hydrogen Sulphide | HNO2 |
| (p) Ammonia | H3BO3 |
| (q) Phosphine | H3PO4 |
| (r) Methane | H2SO4 |
Solution 11.
12.Select the basic radical in the following compounds
a) MgSO4
b) (NH4)2
c) Al2(SO4)3
d) ZnCO3
e) Mg(OH)2
Solution 12.
13. Write the chemical formulae of sulphates of Aliminium, Ammonium and Zinc.
Solution 13.
Valencies of aluminium, ammonium and zinc are 3, 1 and 2, respectively.
The valency of sulphate is 2.
Hence, chemical formulae of the sulphates of aluminium, ammonium and zinc are Al2(SO4)3, (NH4)2SO4 and ZnSO4.
14. The valency of element A is 3 and that of element B is 2. Write the formula of the compound formed by the combination of A and B.
14. The valency of element A is 3 and that of element B is 2. Write the formula of the compound formed by the combination of A and B.
Solution 14.
Formula of the compound = A2B315.Write chemical names of the following compounds
Ca3(PO4)2
K2CO3
K2MnO4
Mn3(BO3)2
Mg(HCO3)2
Na4Fe(CN)6
Ba(Cl)3)2
Ag2SO3
(CH3COO)2Pb
Na2SiO3
Solution 15.
Chemical names of compounds:
Ca3(PO4)2 – Calcium phosphate
K2CO3 – Potassium carbonate
K2MnO4 – Potassium manganate
Mn3(BO3)2 – Manganese (II) borate
Mg(HCO3)2 – Magnesium hydrogen carbonate
Na4Fe(CN)6 – Sodium ferrocyanide
Ba(ClO3)2 – Barium chlorate
Ag2SO3 – Silver sulphite
(CH3COO)2Pb – Lead acetate
Na2SiO3 – Sodium silicate
16. Write the basic and acidic radicals of the following and then write the chemical formulae of these compounds.
a) Barium sulphate
b) Bismuth nitrate
c) calcium bromide
d) Ferrous sulphide
e) Chromium sulphate
f) Calcium silicate
g) Stannic oxide
h) Sodium Zincate
i) Magnesium phosphate
j) Sodium thiosulphate
k) Stannic phosphate
l) Nickel-bi-silphate
m) Potassium mangnate
n) Potassium ferrocynide
16. Write the basic and acidic radicals of the following and then write the chemical formulae of these compounds.
a) Barium sulphate
b) Bismuth nitrate
c) calcium bromide
d) Ferrous sulphide
e) Chromium sulphate
f) Calcium silicate
g) Stannic oxide
h) Sodium Zincate
i) Magnesium phosphate
j) Sodium thiosulphate
k) Stannic phosphate
l) Nickel-bi-silphate
m) Potassium mangnate
n) Potassium ferrocynide
Solution 16.
17.Give the names of the following compounds
a) NaClO
b) NaClO2
c) NaClO3
d) NaClO4
Solution 17.
NaClO – Sodium hypochlorite
NaClO2 – Sodium chlorite
NaClO3 – Sodium chlorate
NaClO4 – Sodium perchlorate
18. Complete the following statements by selecting the correct option.
a)The formula of a compound represents
i) an atom
ii) a particle
iii) a molecule
iv) a combination
18. Complete the following statements by selecting the correct option.
a)The formula of a compound represents
i) an atom
ii) a particle
iii) a molecule
iv) a combination
Solution 18 (a)
iii. The formula of a compound represents a molecule.
b) The correct formula of aluminium oxide is
i) AlO3
ii) AlO2
iii) Al2O3
iv) Al3O2
i) AlO3
ii) AlO2
iii) Al2O3
iv) Al3O2
Solution 18 (b)
iii. The correct formula of aluminium oxide is Al2O3.
c) The valency of Nitrogen in Nitrogen di oxide( NO2) is
i) One
ii) Two
iii) Three
iv) Four
i) One
ii) Two
iii) Three
iv) Four
Solution 18 (c)
iv. The valency of nitrogen in nitrogen dioxide (NO2) is four.
Exercise 1(B)
1.Balance the following equations:
a. Fe + H2O → Fe3O4 + H2
b. Ca + N2 → Ca3N2
c. Zn + KOH → K2ZnO2 + H2
d. Fe2O3 + CO → Fe + CO2
e. PbO + NH3 → Pb + H2O + N2
f. Pb3O4 → PbO + O2
g. PbS + O2 → PbO + SO2
h. S + H2SO4 → SO2 + H2O
i. S + HNO3 → H2SO4 + NO2 + H2O
j. MnO2 + HCl → MnCl2 + H2O + Cl2
k. C + H2SO4 → CO2 + H2O + SO2
l. KOH + Cl2 → KCl + KClO + H2O
m. NO2 +H2O → HNO2 + HNO3
n. Pb3O4 + HCl → PbCl2 + H2O + Cl2
o. H2O + Cl2 → HCl + O2
p. NaHCO3 → Na2CO3 + H2O + CO2
q. HNO3 + H2S → NO2 + H2O + S
r. P + HNO3 → NO2 + H2O + H3PO4
Solution 1.
Balanced chemical equations:
3Fe + 4H2O → Fe3O4 + 4H2
3Ca + N2 → Ca3N2
Zn + 2KOH → K2ZnO2 + H2
Fe2O3 + 3CO → 2Fe + 3CO2
3PbO + 2NH3 → 3Pb + 3H2O + N2
2Pb3O4 → 6PbO + O2
2PbS + 3O2 → 2PbO + 2SO2
S + 2H2SO4 → 3SO2 + 2H2O
S + 6HNO3 → H2SO4 + 6NO2 + 2H2O
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
C + 2H2SO4 → CO2 + H2O + SO2
2KOH + Cl2 → KCl + KClO + H2O
2NO2 + H2O → HNO2 + HNO3
Pb3O4 + 8HCl → 3PbCl2 + 4H2O + Cl2
2H2O + 2Cl2 → 4HCl + O2
2NaHCO3 → Na2CO3 + H2O + CO2
2HNO3 + H2S → 2NO2 + 2H2O + S
P + 5HNO3 → 5NO2 + H2O + H3PO4
Exercise 1(C)
1. Fill in the blanks:
(a) Dalton used symbol _____ for oxygen _____ for hydrogen.
(b) Symbol represents _____ atom(s) of an element.
(c) Symbolic expression for a molecule is called _____. .
(d) Sodium chloride has two radicals. Sodium is a _____ radical while chloride is _____ radical.
(e) Valency of carbon in CH4 is _____ , in C2H6 _____, in C2H4 ___ and in C2H2 is ____.
(f) Valency of Iron in FeCl2 is _____ and in FeCl3 it is ____ .
(g) Formula of iron (ill) carbonate is _____ .
Solution 1.
2. Complete the following table.
Solution 2.
Acid Radicals

Basic Radicals
|
Chloride
|
Nitrate
|
Sulphate
|
Carbonate
|
Hydroxide
|
Phosphate
|
Magnesium
|
MgCl2
|
Mg(NO3)2
|
MgSO4
|
MgCO3
|
Mg(OH)2
|
Mg3(PO4)2
|
Sodium
| ||||||
Zinc
| ||||||
Silver
| ||||||
Ammonium
| ||||||
Calcium
| ||||||
Iron (II)
| ||||||
Potassium
|
Solution 2.
3. Sodium chloride reacts with silver nitrate to produce silver chloride and sodium nitrate
Write the equation.
Check whether it is balanced, if not balance it.
Find the weights of reactants and products.
State the law which this equation satisfies.
Solution 3.
Write the equation.
Check whether it is balanced, if not balance it.
Find the weights of reactants and products.
State the law which this equation satisfies.
Solution 3.
(a) NaCl+ AgNO3 → NaNO3 + AgCl↓
(b) It is a balanced equation.
(c) Weights of reactants:NaCl – 58.44, AgNO3 – 169.87
Weights of products: NaNO3 – 84.99, AgCl – 143.32
NaCl + AgNO3 → NaNO3 + AgCl
(23+35.5) + (108+14+48) → (23+14+48) + (108+35.5)
58.5 + 170 → 85 + 143.5
228.5 g → 228.5 g
(d) Law of conservation of mass: Matter is neither created nor destroyed in the course of a chemical reaction.
4. What information does the following chemical equation convey?
(a) Zn + H2SO4 → ZnSO4+ H2
(b) Mg + 2HCl → MgCl2+ H2
Solution 4
(a) This equation conveys the following information:
- The actual result of a chemical change.
- Substances take part in a reaction, and substances are formed as a result of the reaction.
- Here, one molecule of zinc and one molecule of sulphuric acid react to give one molecule of zinc sulphate and one molecule of hydrogen.
- Composition of respective molecules, i.e. one molecule of sulphuric acid contains two atoms of hydrogen, one atom of sulphur and four atoms of oxygen.
- Relative molecular masses of different substances, i.e. molecular mass of
Zn = 65
H2SO4 = (2+32+64) = 98
ZnSO4 = (65+32+64) = 161
H2 = 2
6. 22.4 litres of hydrogen are formed at STP.
(b) This equation conveys the following information:
- Magnesium reacts with hydrochloric acid to form magnesium chloride and hydrogen gas.
- 24 g of magnesium reacts with 2(1 + 35.5) = 73 g of hydrochloric acid to produce (24 + 71), i.e. 95 g of magnesium chloride.
- Hydrogen produced at STP is 22.4 litres.
5.(a) What are poly atomic ions? Give two examples.
(b) Name the fundamental law that is involved in every equation.
(b) Name the fundamental law that is involved in every equation.
Solution 5
(a) A poly-atomic ion is a charged ion composed of two or more atoms covalently bounded that can be carbonate (CO32-) and sulphate (SO42-)
(b) The fundamental laws which are involved in every equation are:
- A chemical equation consists of formulae of reactants connected by plus sign (+) and arrow (→) followed by the formulae of products connected by plus sign (+).
- The sign of an arrow (→) is to read ‘to form’. It also shows the direction in which reaction is predominant.
6. What is the valency of :
(a) fluorine in CaF2
(b) sulphur in SF6
(c) phosphorus in PH3
(d) carbon in CH4
(e) nitrogen in the following compounds:
(i) N2O3 (ii) N2O5 (iii) NO2 (iv) NO
(a) fluorine in CaF2
(b) sulphur in SF6
(c) phosphorus in PH3
(d) carbon in CH4
(e) nitrogen in the following compounds:
(i) N2O3 (ii) N2O5 (iii) NO2 (iv) NO
Solution 6.
(a) Valency of fluorine in CaF2 is -1.
(b) Valency of sulphur in SF6 is -6.
(c) Valency of phosphorus in PH3 is +3.
(d) Valency of carbon in CH4 is +4.
(e) Valency of nitrogen in the given compounds:
- N2O3 = N is +3
- N2O5 = N is +5
- NO2 = N is +4
- NO = N is +2
7. Why should an equation be balanced? Explain with the help of a simple equation.
Solution 7.
According to law of conservation of mass, “matter can neither be created nor be destroyed in a chemical reaction”. This is possible only, if total number of atoms on the reactants side is equals to total number of atoms on products side. Thus, a chemical reaction should be always balanced.
Let us consider an example,
Fe + H2O → Fe3O4 + H2
In this equation number of atoms on both sides is not the same, the equation is not balanced.
The balanced form of this equation is given by,
3Fe + 4H2O → Fe3O4 + 4H2
8.Write the balanced chemical equations of the following reactions.
(a)sodium hydroxide + sulphuric acid → sodium sulphate + water
(b) potassium bicarbonate + sulphuric acid → potassium sulphate + carbon dioxide + water
(c) iron + sulphuric acid → ferrous sulphate + hydrogen.
(d) chlorine + sulphur dioxide + water → sulphuric acid + hydrogen chloride
(e) silver nitrate → silver + nitrogen dioxide + oxygen
(f) copper + nitric acid → copper nitrate + nitric oxide + water
(g) ammonia + oxygen → nitric oxide + water
(h) barium chloride + sulphuric acid → barium sulphate + hydrochloric acid
(i) zinc sulphide + oxygen → zinc oxide + sulphur dioxide
(j) aluminium carbide + water → aluminium hydroxide + methane
(k) iron pyrites(FeS2) + oxygen → ferric oxide + sulphur dioxide
(l) potassium permanganate + hydrochloric acid → potassium chloride + manganese chloride + chlorine + water
(m) aluminium sulphate + sodium hydroxide → sodium sulphate + sodium meta aluminate + water.
(n) aluminium + sodium hydroxide + water → sodium meta aluminate + hydrogen
(o) potassium dichromate + sulphuric acid → potassium sulphate + chromium sulphate + water + oxygen
(p) potassium dichromate + hydrochloric acid → Potassium chloride + chromium chloride + water + chlorine
(q) sulphur + nitric acid → sulphuric acid + nitrogen dioxide + water
(r) potassium iodide + manganese dioxide + sulphuric acid → iodine + potassium bisulphate + manganese sulphate + water
Solution 8.
9.
(a) Define atomic mass unit.
1gm mass and 1kg mass =? a.m.u
(b)Calculate the molecular mass of the following:
Given atomic mass of Cu = 63·5, H = 1, O= 16, C = 12, N = 14, Mg = 24, S = 32
1.CuSO45H2O
2.(NH4)2CO3
3.(NH2)2CO
4.Mg3N2
Solution 9.
(a) The atomic mass unit (amu) is defined as 1/12th of the mass of an atom of carbon.
1 a.m.u. = 1.67 x 10-24 gm = 1.67 x 10-27 kg
1 gm mass = 6.02 x 1023 a.m.u. and 1 kg mass = 6.02 x 1026 a.m.u.
(b)
(b)
1. The relative molecular mass of = CuSO4 . 5H2O
= 63.5 + 32 + (16 x 4) + 5 (2 + 16)
= 159.5 + 90 = 249.5
2.The relative molecular mass of = (NH4)2CO3 = N2H8CO3
= 14 x 2 + 1 x 8 + 12 + 3 x 16
= 28 + 8 + 12 + 48 = 96
3.The relative molecular mass of = (NH2)2CO = N2H4CO
= 2 x 14 + 1 x 4 + 12 + 16
= 28 + 4 + 12 + 16 = 60
4.The relative molecular mass of = Mg3N2 = 3 x 24 + 2 x 14 = 72 + 28 = 100
10.Multiple Choice Type
(a)Modern atomic symbols are based on the method proposed by
(i) Bohr
(ii) Dalton
(iii) Berzelius
(iv) Alchemist
(b)The number of carbon atoms in a hydrogen carbonate radical is
(i) One
(ii) Two
(iii) Three
(iv) Four
(c)The formula of iron (III) sulphate is
(i) Fe3SO4
(ii) Fe(SO4)3
(iii) Fe2(SO4)3
(iv) FeSO4
(d)In water, the hydrogen-to-oxygen mass ratio is
(i) 1: 8
(ii) 1: 16
(iii) 1: 32
(iv) 1: 64
(e)The formula of sodium carbonate is Na2CO3 and that of calcium hydrogen carbonate is
(i) CaHCO3
(ii) Ca(HCO3)2
(iii) Ca2HCO3
(iv) Ca(HCO3)3
Solution 10.
(a) (iii) Berzelius
(b) (i) One
(c) (iii) Fe2(SO4)3
(d) (i) 1: 8
(e) (ii) Ca(HCO3)2
11.Correct the following statements
(a) A molecular formula represents an element
(b) Molecular formula of water is H2O2.
(c)
(d) CO and Co both represents cobalt.
11.Correct the following statements
(a) A molecular formula represents an element
(b) Molecular formula of water is H2O2.
(c)
(d) CO and Co both represents cobalt.
Solution 11.
(a) A molecular formula represent The Molecule of an element or of a Compound.
(b) The molecular formula of water (H2O) represents 18 parts by mass of water.
(c) A balanced equation obeys the law of conservation of mass wherever unbalanced equation does not obey this law.
(d) CO and Co represent carbon-monoxide and cobalt respectively.
12. Calculate the molecular masses of the following:
1. CHCl3
2. (NH4)2 Cr2O7
3. (NH4)2SO4
4. CH3COONa
5. (KClO3)
6. (NH4)2PtCl6
[At.mass C= 12, H=1, Cl= 35.5, N=14, Cr=52, O=16, S=32, Na=23, K=39.1, Pt=195.08]
12. Calculate the molecular masses of the following:
1. CHCl3
2. (NH4)2 Cr2O7
3. (NH4)2SO4
4. CH3COONa
5. (KClO3)
6. (NH4)2PtCl6
[At.mass C= 12, H=1, Cl= 35.5, N=14, Cr=52, O=16, S=32, Na=23, K=39.1, Pt=195.08]
Solution 12.
1. Relative molecular mass of CHCl3
= 12 + 1 + (3 × 35.5)
= 12 + 1 + 106.5
= 119.5
2. Relative molecular mass of (NH4)2 Cr2O7
= (14 × 2) + (1× 8) + (52 × 2) + (16 × 7)
= 28 + 8 + 104 + 112
= 252
3. Relative molecular mass of (NH4)2SO4
= (2 × 14) + (8 × 1) + 32 + (4 × 16)
= 28 + 8 + 32 + 64
= 132
4. Relative molecular mass of CH3COONa
= (12 × 2) + (1× 3) + (16 × 2) + 23
= 24 + 3 + 32 + 23
= 82
5. Potassium chlorate (KClO3)
= 39.1+ 35.5 + (16 × 3)
= 39.1+ 35.5 + 48
= 122.6
6. Ammonium chloroplatinate (NH4)2PtCl6
= (14 × 2) + (1 × 8) + 195.08 + (35.5 × 6)
= 28 + 8 + 195.08 + 213
= 444.08
13.Give the empirical formula of :
(a) Benzene(C6H6)
(b) Glucose (C6H12O6)
(c) Acetylene (C2H2)
(d) Acetic acid (CH3COOH)
13.Give the empirical formula of :
(a) Benzene(C6H6)
(b) Glucose (C6H12O6)
(c) Acetylene (C2H2)
(d) Acetic acid (CH3COOH)
Solution 13.
14.Find the percentage mass of water in Epsom salt MgSO4·7H2O
Solution 14.
Solution 14.
Relative molecular mass of MgSO4·7H2O
=24 + 32 + (16 × 4) + 7(2 + 16)
=24 + 32 + 64 + 126
=246
26 g of Epsom salt contains 126 g of water of crystallisation.
Hence, 100 g of Epsom salt contains
The % of H2O in MgSO4·7H2O = 51.2
15. Calculate the percentage of phosphorus in:
(a) Calcium hydrogen phosphate Ca(H2PO4)2
(b) Calcium phosphate Ca3(PO4)2
Solution 15.
(a) Calcium hydrogen phosphate Ca(H2PO4)2
(b) Calcium phosphate Ca3(PO4)2
Solution 15.
(a) Relative molecular mass of Ca(H2PO4)2
= 40.07 + (1 × 4) + (30.9 × 2) + (16 × 8)
= 40.07 + 4 + 61.8 + 128
= 233.87
233.87 g Ca(H2PO4)2 contains 61.8 g P
So, 100 g Ca(H2PO4)2 contains
The % of P in Ca(H2PO4)2 is 26.42%.
(b) Relative molecular mass of Ca3(PO4)2
= (40.07 × 3) + (30.9 × 2) + (16 × 8)
= 120.21 + 61.8 + 128
= 310.01
310.01 g Ca3(PO4)2 contains 61.8 g P
So, 100 g Ca(H2PO4)2 contains
(IMAGE)
The % of P in Ca(H2PO4)2 is 19.93%.
16. Calculate the percentage composition of each element in Potassium chlorate ,KClO3
.
Solution 16.
Relative molecular mass of KClO3
= 39.09 + 35.5 + (3 × 16)
= 122.59 g
The percentages of K, Cl and O in KClO3 are 31.9%, 28.9% and 39.1%, respectively.
17. Urea is a very important nitrogenous fertilizer. Its formula is CON,H4. Calculate the percentage of carbon in urea .(C=12, O=16,N=14 and H=1)
Solution 17.
Relative molecular mass of urea is
Element No. of atoms Atomic mass Total
N 2 14 28
C 1 12 12
H 4 1 4
O 1 16 16
[12 + 16 + 28 + 4] = 60
Hence, relative molecular mass of urea = 60
Selina ICSE Solutions for Class 9 Chemistry Chapter 1 - The Language of Chemistry